Synthesis and Characterization of Organochalcogens (S, Te, Se)

DOI:
https://doi.org/10.54060/a2zjournals.jase.80Keywords:
Organochalcogens, Sulfur, Selenium, Tellurium, Synthesis, CharacterizationAbstract
Organochalcogen compounds, which include sulfur (S), tellurium (Te), and selenium (Se), constitute a diverse and highly functional class of molecules with broad applications in medicinal chemistry, materials science, and catalysis. This review explores various synthetic approaches for organochalcogens, such as nucleophilic substitution, transition metal-catalyzed coupling, and cyclization reactions, aiming to enhance reaction efficiency and sustainability. Notably, microwave-assisted synthesis has emerged as an eco-friendly alternative, significantly reducing reaction time while enhancing product selectivity. The preparation of organotellurium(IV) diiodides and dithiocarbamate-functionalized tellurium compounds underscores their structural flexibility and potential in coordination chemistry. In organosulfur chemistry, the development of dithiocarbamate-functionalized organotellurium(IV) derivatives has demonstrated high reaction efficiency, achieving yields of up to 93%, with monodentate ligand coordination confirmed by spectroscopic techniques. Additionally, selenium-containing heterocycles, including selenenophenes, selenochromenes, and quinoxalines, have been synthesized via photoinduced cyclization and radical pathways, often facilitated by copper(I) iodide, iron(III) chloride, or dialkyl diselenides. Despite these advancements, challenges persist in improving compound stability, optimizing green synthetic methods, and broadening their functional applications. Furthermore, the study confirms the formation of stable organochalcogen derivatives with distinct structural and electronic properties, demonstrating potential applications in antioxidant, anticancer, and photovoltaic technologies.
Downloads
References
A. T. Odularu and P. A. Ajibade, “Dithiocarbamates: challenges, control, and approaches to excellent yield, characteriza-tion, and their biological applications,” Bioinorganic Chemistry and Applications, vol. 2019, pp. 1–15, 2019. doi:10.1155/2019/8260496.
M. Sharma, A. Sharma, and R. Sachar, “Synthesis and characterization of the adducts of morpholinedithiocarbamate com-plexes of oxovanadium (IV), nickel (II), and copper (II) with piperidine and morpholine,” E-Journal of Chemistry, vol. 9, no. 4, pp. 1929–1940, 2012. doi:10.1155/2012/689501.
G. Hogarth, “Metal-dithiocarbamate complexes: chemistry and biological activity,” Mini-Reviews in Medicinal Chemistry, vol. 12, no. 12, pp. 1202–1215, 2012. doi:10.2174/138955712802762095.
L. A. Ramos and E. T. G. Cavalheiro, “Preparation, characterization and thermal decomposition of sodium and potassium salts of dithiocarbamate,” Brazilian Journal of Thermal Analysis, vol. 2, no. 1, pp. 38–44, 2014. doi:10.18362/bjta.v2i1.11.
C. Maurya and S. Bajpai, “Biological applications of metal complexes of dithiocarbamates,” Journal of Applied Science and Education, vol. 2, no. 1, pp. 1–16, 2022. doi:10.54060/JASE/002.01.002.
P. J. Nieuwenhuizen, A. W. Ehlers, J. G. Haasnoot, et al., “The mechanism of zinc(II)-dithiocarbamate accelerated vulcani-zation uncovered; theoretical and experimental evidence,” Journal of the American Chemical Society, vol. 121, no. 1, pp. 163–168, 1999. doi:10.1021/ja982217n.
A. Henckens, K. Colladet, S. Fourier, et al., “Synthesis of 3,4-diphenyl-substituted poly(thienylenevinylene), low-bandgap polymers via the dithiocarbamate route,” Macromolecules, vol. 38, no. 1, pp. 19–26, 2005. doi:10.1021/ma047940e.
V. Orescanin, L. Mikelic, V. Roje, et al., “Determination of lanthanides by source-excited energy dispersive X-ray fluores-cence (EDXRF) method after preconcentration with ammonium pyrrolidinedithiocarbamate (APDC),” Analytica Chimica Acta, vol. 570, no. 2, pp. 277–282, 2006. doi:10.1016/j.aca.2006.04.028.
A. Jayaraju, K. Rameshbabu, and J. Sreeramulu, “Synthesis, characterization and biological evaluation of histamine dithi-ocarbamate metal complexes,” International Journal of Pharmacy and Pharmaceutical Research, vol. 4, no. 2, pp. 241–247, 2015. Available from: https://ijppr.humanjournals.com/21-2/.
X. Hou, X. Li, H. Hemit, et al., “Synthesis, characterization, and antitumor activities of new palladium(II) complexes with 1-(alkyldithiocarbonyl)-imidazoles,” Journal of Coordination Chemistry, vol. 67, no. 3, pp. 461–469, 2014. doi:10.1080/00958972.2014.890717.
H. Mansouri-Torshizia, M. Saeidifar, A. Divsalar, et al., “Interaction studies between a 1,10-phenanthroline adduct of pal-ladium(II) dithiocarbamate anti-tumor complex and calf thymus DNA. A synthesis spectral and in-vitro study,” Spectro-chimica Acta Part A: Molecular and Biomolecular Spectroscopy, vol. 77, pp. 312–318, 2010. doi:10.1016/j.saa.2010.05.029.
A. J. Odola and J. A. O. Woods, “New Ni(II) mixed ligand complexes of dithiocarbamates with Schiff base,” Journal of Chemical and Pharmaceutical Research, vol. 3, no. 6, pp. 865–871, 2011. Available from: www.jocpr.com.
G. Barone, T. Chaplin, T. G. Hibbert, et al., “Synthesis and thermal decomposition studies of homo- and heteroleptic tin(IV) thiolates and dithiocarbamates: molecular precursors for tin sulfides,” Journal of the Chemical Society, Dalton Transactions, vol. 6, pp. 1085–1092, 2002. doi:10.1039/b108509n.
D. Buac, S. Schmitt, G. Ventro, et al., “Dithiocarbamate based coordination compounds as potent proteasome inhibitors in human cancer cells,” Mini-Reviews in Medicinal Chemistry, vol. 12, no. 12, pp. 1193–1201, 2012. doi:10.2174/138955712802762040.
C. K. Adokoh, “Therapeutic potential of dithiocarbamate supported gold compounds,” RSC Advances, vol. 10, pp. 2975–2988, 2020. doi:10.1039/C9RA09682E.
A. J. Odola and J. A. O. Woods, “Synthesis, characterization and antimicrobial activity studies of new nickel(II) mixed ligand complexes of disubstituted dithiocarbamates with ethylsalicylaldiminate,” Archives of Applied Science Research, vol. 3, no. 4, pp. 463–470, 2011. Available from: www.scholarsresearchlibrary.com.
R. A. Kamoon, S. A. Nadhum, and M. H. Mohammed, “Dithiocarbamates derivatives as anticancer agents: a review,” An-nals of Tropical Medicine and Public Health, vol. 23, no. 19, pp. 232–113, 2020. doi:10.36295/ASRO.2020.232113.
J. O. Adeyemi and D. C. Onwudiwe, “Organotin(IV) dithiocarbamate complexes: chemistry and biological activity,” Mole-cules, vol. 23, no. 10, pp. 1–27, 2018. doi:10.3390/molecules23102571.
C. Marzano, L. Ronconi, F. Chiara, et al., “Gold(III)-dithiocarbamato anticancer agents: activity, toxicology and histopatho-logical studies in rodents,” International Journal of Cancer, vol. 129, no. 2, pp. 487–496, 2011. doi:10.1002/ijc.25684.
C. Maurya, “Glimpses of synthesis and biological applications of metal-dithiocarbamate derivatives,” International Journal of Scientific Research, vol. 11, no. 7, pp. 876–886, 2022. doi:10.21275/SR22708193015.
A. Kumar, G. K. Rao, F. Saleem, et al., “Efficient catalysis of Suzuki–Miyaura C–C coupling reactions with palladium(II) com-plexes of partially hydrolyzed bisimine ligands: a process important in environmental context,” Journal of Hazardous Ma-terials, vol. 269, pp. 9–17, 2014. doi:10.1016/j.jhazmat.2013.11.024.
P. Oswal, A. Arora, S. Purohit, et al., “Metal-metalloid bond containing complexes of the bulky organotellurium ligand: applications in catalysis of C–O coupling and aldehyde to amide transformation reactions,” New Journal of Chemistry, vol. 47, pp. 4346–4354, 2023. doi:10.1039/D2NJ04408K.
A. Arora, P. Oswal, D. Sharma, et al., “Organosulphur, organoselenium, and organotellurium compounds for the develop-ment of heterogeneous and nanocatalytic systems for Suzuki coupling,” Dalton Transactions, vol. 51, no. 5, pp. 17114–17144, 2022. doi:10.1039/D2DT02558B.
A. Arora, P. Oswal, D. Sharma, et al., “Molecular organosulphur, organoselenium, and organotellurium complexes as ho-mogeneous transition metal catalytic systems for Suzuki coupling,” ChemistrySelect, vol. 7, no. 33, e202201704, 2022. doi:10.1002/slct.202201704.
P. Oswal, A. Arora, S. Gairola, et al., “Organosulfur, organoselenium, and organotellurium ligands in the development of palladium, nickel, and copper-based catalytic systems for Heck coupling,” New Journal of Chemistry, vol. 45, pp. 21449–21487, 2021. doi:10.1039/D1NJ02971A.
A. Arora, P. Oswal, A. Datta, et al., “Complexes of metals with organotellurium compounds and nanosized metal tellurides for catalysis, electrocatalysis, and photocatalysis,” Coordination Chemistry Reviews, vol. 459, 214406, 2022. doi:10.1016/j.ccr.2022.214406.
A. Arora, P. Oswal, G. K. Rao, et al., “Tellurium-ligated Pd(II) complex of bulky organotellurium ligand as a catalyst of Suzu-ki coupling: first report on in situ generation of bimetallic alloy ‘telluropalladinite’ (Pd₉Te₄) nanoparticles and role in highly efficient catalysis,” Catalysis Letters, vol. 152, no. 7, pp. 1999–2011, 2022. doi:10.1007/s10562-021-03769-4.
A. Arora, P. Oswal, G. K. Rao, et al., “Catalytically active nanosized Pd₉Te₄ (telluropalladinite) and PdTe (kotulskite) alloys: first precursor-architecture controlled synthesis using palladium complexes of organotellurium compounds as single source precursors,” RSC Advances, vol. 11, pp. 7214–7224, 2021. doi:10.1039/D0RA08732G.
P. Singh, D. Das, A. Kumar, et al., “Palladium(II) complexes of N-2-(aryltelluro)ethylmorpholine/piperidine: synthesis, structure, application in Heck coupling and unprecedented conversion into nano-sized PdTe,” Inorganic Chemistry Com-munications, vol. 15, pp. 163–166, 2012. doi:10.1016/j.inoche.2011.10.015.
R. Saxena and P. Sharma, “Antibacterial and antifungal evaluation of some chalcogen-bearing ligands, their transition and non-transition metal complexes,” Indian Journal of Pharmaceutical and Biological Research, vol. 3, no. 3, pp. 1–6, 2015. doi:10.30750/ijpbr.3.3.1.
R. F. Al-Asadi, “Synthesis, DFT calculation, and biological activity of some organotellurium compounds containing azome-thine group,” Orbital: Electron Journal of Chemistry, vol. 11, no. 7, pp. 1–10, 2019. doi:10.17807/orbital.v11i7.1211.
W. A. Al-Masoudi, R. H. Al-Asadi, R. M. Othman, et al., “Synthesis, antimicrobial activity, computational and modeling studies of some new organotellurium compounds containing azo moieties,” European Journal of Chemistry, vol. 6, no. 4, pp. 374–380, 2015. doi:10.5155/eurjchem.6.4.374-380.1254.
H. A. Stefani, G. V. Botteselle, J. Zukerman-Schpector, et al., “Synthesis, anti-inflammatory activity, and molecular docking studies of 2,5-diarylfuran amino acid derivatives,” European Journal of Medicinal Chemistry, vol. 47, pp. 52–58, 2012. doi:10.1016/j.ejmech.2011.10.018.
T. Paschoalin, A. A. Martens, A. T. Omori, et al., “Antitumor effect of chiral organotelluranes elicited in a murine melano-ma model,” Bioorganic & Medicinal Chemistry, vol. 27, no. 12, pp. 2537–2545, 2019. doi:10.1016/j.bmc.2019.03.032.
X.-Q. Zeng, H.-T. Tian, F.-H. Chen, et al., “Electrocatalytic three-component reactions: synthesis of tellurium-containing oxazolidinone for anticancer agents,” Green Chemistry, vol. 25, pp. 5024–5029, 2023. doi:10.1039/D3GC01288C.
A. M. V. Nunes, F. D. C. Pereira de Andrade, L. A. Filgueiras, et al., “preADMET analysis and clinical aspects of dogs treated with the organotellurium compound RF07: a possible control for canine visceral leishmaniasis?,” Environmental Toxicology and Pharmacology, vol. 80, 103470, 2020. doi:10.1016/j.etap.2020.103470.
R. H. Al-Asadi, W. A. Al-Masoudi, and K. S. Abdual-Rassol, “Synthesis, biological activity, and computational study of some new unsymmetrical organotellurium compounds derived from 2-amino-5-carboxyphenyl mercury(II) chloride,” Asian Jour-nal of Chemistry, vol. 28, no. 6, pp. 1171–1176, 2016. doi:10.14233/ajchem.2016.19139.
C. Maurya, S. Bajpai, P. K. Kushwaha, U. Chand, and S. Singh, “Synthesis, spectroscopic characterization, and in vitro anti-microbial study of novel organotellurium (IV) diphenyldithiocarbamate derivatives,” Journal of Sulfur Chemistry, vol. 45, no. 4, pp. 477–489, 2024. doi:10.1080/17415993.2024.2360441.
J. Yu, D. Qi, and J. Li, “Study on chemical communication,” Communications Chemistry, vol. 3, p. 189, 2020. doi:10.1038/s42004-020-00438-2.
M. S. Thakur, N. Singh, A. Sharma, R. Rana, A. R. Abdul Syukor, M. Naushad, S. Kumar, M. Kumar, and L. Singh, “Coordina-tion chemistry review,” Coordination Chemistry Reviews, vol. 471, 214739, 2022. doi:10.1016/j.ccr.2022.214739.
X. Yu and D. Sun, “Synthesis and properties of organic molecules,” Molecules, vol. 18, p. 6230, 2013. doi:10.3390/molecules18066230.
B. D. Nath, K. Takaishi, and T. Ema, “Advances in catalytic science and technology,” Catalysis Science & Technology, vol. 10, p. 12, 2020. doi:10.1039/C9CY01894H.
M. Yadav, D. Yadav, D. P. Singh, and J. K. Kapoor, “Synthesis and characterization of inorganic compounds,” Inorganica Chimica Acta, vol. 546, 121300, 2023. doi:10.1016/j.ica.2022.121300.
J. Seto, S. Tamura, N. Asai, N. Kishii, Y. Kijima, and N. Matsuzawa, “Chemical studies in pure and applied chemistry,” Pure and Applied Chemistry, vol. 68, p. 1429, 1996. doi:10.1351/pac199668071429.
D. Xia, P. Wang, X. Ji, N. M. Khashab, J. L. Sessler, and F. Huang, “Comprehensive chemical review,” Chemical Reviews, vol. 120, p. 6070, 2020. doi:10.1021/acs.chemrev.9b00839.
A. Chaudhary and E. Rawat, “Research in inorganic chemistry,” International Journal of Inorganic Chemistry, vol. 2014, 509151, 2014. doi:10.1155/2014/509151.
Q. He, G. I. Vargas-Zúñiga, S. H. Kim, S. K. Kim, and J. L. Sessler, “Advancements in chemical reviews,” Chemical Reviews, vol. 119, p. 9753, 2019. doi:10.1021/acs.chemrev.8b00734.
M. T. Chaudhry, S. Akine, and M. J. MacLachlan, “Review on chemical societies,” Chemical Society Reviews, vol. 50, p. 10713, 2021. doi:10.1039/D1CS00225B.
J. Grajewski, “Molecular structure and reactivity,” Molecules, vol. 27, p. 1004, 2022. doi:10.3390/molecules27031004.
A. Gulino, P. Dapporto, P. Rossi, and I. Fragalà, “Material chemistry advancements,” Chemistry of Materials, vol. 14, p. 4955, 2002. doi:10.1021/cm021183m.
Y. Cheng, T. J. Emge, and J. G. Brennan, “Structural analysis of inorganic compounds,” Inorganic Chemistry, vol. 35, p. 7339, 1996. doi:10.1021/ic9603969.
N. Kushwah, G. Kedarnath, A. Wadawale, K. K. Halankar, B. P. Mandal, M. Jafar, and B. Vishwanadh, “Synthesis and prop-erties of new inorganic materials,” Inorganic Chemistry, vol. 62, p. 8823, 2023. doi:10.1021/acs.inorgchem.3c00269.
Y. Nishibayashi, K. Segawa, J. D. Singh, S. Fukuzawa, K. Ohe, and S. Uemura, “Organometallic chemistry studies,” Organo-metallics, vol. 15, p. 370, 1996. doi:10.1021/om950533u.
Y. Nishibayashi, J. D. Singh, Y. Arikawa, S. Uemura, and M. Hidai, “Transition metal catalysis in organometallic chemistry,” Journal of Organometallic Chemistry, vol. 531, p. 13, 1997. doi:10.1016/S0022-328X(96)06681-8.
M. Kamboj, “Advances in physical science research,” Physical Sciences Reviews, vol. 8, p. 4541, 2023. doi:10.1515/psr-2021-0106.
A. Panda, S. C. Menon, H. B. Singh, C. P. Morley, R. Bachman, T. M. Cocker, and R. J. Butcher, “European studies in inor-ganic chemistry,” European Journal of Inorganic Chemistry, vol. 2005, p. 1114, 2005. doi:10.1002/ejic.200400757.
A. J. Barton, A. R. J. Genge, N. J. Hill, W. Levason, S. D. Orchard, B. Patel, G. Reid, and A. J. Ward, “Heteroatom chemistry research,” Heteroatom Chemistry, vol. 13, p. 550, 2002. doi:10.1002/hc.10100.
Nitu and K. K. Verma, “Electronic journal of chemistry studies,” E-Journal of Chemistry, vol. 8, p. 1158, 2011. doi:10.1155/2011/768192.
N. Rathee and K. Verma, “Chemical research in Serbian science,” Journal of the Serbian Chemical Society, vol. 77, p. 325, 2012. doi:10.2298/JSC101211200R.
S. Kumari, K. Verma, and S. Garg, “Studies in international chemistry,” International Journal of Chemical Sciences, vol. 15, p. 207, 2017.
S. Kumari and S. Garg, “Recent advancements in chemical sciences,” Chemical Science Transactions, vol. 8, p. 48, 2019. doi:10.7598/cst2019.1556.
C. Maurya, S. Bajpai, P. K. Kushwaha, U. Chand, and S. Singh, “Synthesis, characterization and in vitro antimicrobial activity of organotellurium decorated 10-membered tetraazamacrocyclic complexes of cobalt(II),” Asian Journal of Chemistry, vol. 36, no. 5, pp. 1056–1060, 2024. doi:10.14233/ajchem.2024.31358.
P. T. Anastas and J. C. Warner, “Principles of green chemistry,” Green Chemistry: Theory and Practice, vol. 29, pp. 14821–14842, 1998.
R. B. N. Baig and R. S. Varma, “Alternative energy input: Mechanochemical, microwave, and ultrasound-assisted organic synthesis,” Chemical Society Reviews, vol. 41, no. 4, pp. 1559–1584, 2012. doi:10.1039/CICS15204A.
V. Santagada, F. Frecentese, E. Perissutti, F. Fiorino, B. Severino, and G. Caliendo, “Microwave-assisted synthesis: A new technology in drug discovery,” Mini Reviews in Medicinal Chemistry, vol. 9, no. 3, pp. 340–358, 2009. doi:10.2174/1389557510909030340.
S. Tiwari and S. Talreja, “Green chemistry and microwave irradiation Unloa technique: A review,” Journal of Pharmaceutical Research International, vol. 34, no. 39A, pp. 74–79, 2022. doi:10.9734/jpri/2022/v34139A36240.
D. Garella, E. Borretto, A. Di Stilo, K. Martina, G. Cravotto, and P. Cintas, “Microwave-assisted synthesis of N-heterocycles in medicinal chemistry,” MedChemComm, vol. 4, no. 10, pp. 1323–1343, 2013. doi:10.1039/c3md00152k.
M. Colombo and I. Peretto, “Chemistry strategies in early drug discovery: An overview of recent trends,” Drug Discovery Today, vol. 13, no. 15–16, pp. 677–684, 2008.
S. Takkellapati, “Microwave-assisted chemical transformations,” Current Perspectives in Organic Chemistry, vol. 17, no. 20, pp. 2305–2322, 2013. doi:10.2174/13852728113179990042.
M. Aranda, A. I. Moreno, F. P. Cossío, M. de Victoria Gómez, A. Cózar, Á. Díaz-Ortiz, A. de la Hoz, and P. Prieto, “Micro-wave-assisted reactions of nitroheterocycles with dienes: Diels-Alder and tandem hetero Diels-Alder/[3,3] sigmatropic shift,” Tetrahedron, vol. 65, no. 27, pp. 5328–5336, 2009. doi:10.1016/j.tet.2009.04.065.
B. Martinez-Gualda, S. Y. Pu, M. Froeyen, P. Herdewijn, S. Einav, and S. De Jonghe, “Structure-activity relationship study of isothiazolo[4,3-b]pyridines as antiviral agents,” Bioorganic & Medicinal Chemistry, vol. 28, p. 115188, 2020. doi:10.1016/j.bmc.2019.115188.
D. Bayramoğlu, G. Gürel, A. Sinağ, and M. Güllü, “Thermal conversion of glycerol to value-added chemicals: Pyridine de-rivatives by one-pot microwave-assisted synthesis,” Turkish Journal of Chemistry, vol. 38, no. 4, p. 670, 2014. doi:10.3906/kim-1312-47.
H. M. Abd El-Lateef, A. A. Abdelhamid, M. M. Khalaf, M. Gouda, N. A. A. Elkanzi, H. El-Shamy, and A. M. Ali, “Green syn-thesis of novel pyridines via one-pot multicomponent reaction and their anti-inflammatory evaluation,” ACS Omega, vol. 8, no. 12, pp. 11326–11334, 2023. doi:10.1021/acsomega.3c00066.
K. Shekarrao, P. P. Kaishap, V. Saddanapu, A. Addlagatta, S. Gogoi, and R. C. Boruah, “Microwave-assisted palladi-um-mediated efficient synthesis of pyrazolo[3,4-b]pyridines and related heterocycles,” RSC Advances, vol. 4, no. 46, pp. 24001–24006, 2014. doi:10.1039/C4RA02865A.
D. Amariucai-Mantu, V. Mangalagiu, R. Danac, and I. I. Mangalagiu, “Microwave assisted reactions of azaheterocycles for medicinal chemistry applications,” Molecules, vol. 25, no. 3, p. 716, 2020. doi: 10.3390/molecules25030716.
S. Mehta, N. Swarnkar, R. Vyas, J. Vardia, P. B. Punjabi, and S. C. Ameta, “Microwave assisted synthesis of some pyridine derivatives containing mercaptotriazole and thiazolidinone as a new class of antimicrobial agents,” Phosphorus Sulfur Sili-con Relat. Elem., vol. 183, no. 1, pp. 105–114, 2007. doi: 10.1080/10426500701557138.
C. Maurya, S. Bajpai, P. K. Kushwaha, U. Chand, and S. Singh, “Synthesis, spectroscopic characterization, and in vitro anti-microbial study of novel organotellurium (IV) diphenyldithiocarbamate derivatives,” J. Sulfur Chem., vol. 45, no. 4, pp. 477–489, 2024. doi: 10.1080/17415993.2024.2360441.
C. Maurya, S. Bajpai, P. K. Kushwaha, U. Chand, and S. Singh, “Synthesis, characterization and in vitro antimicrobial activity of organotellurium decorated 10-membered tetraazamacrocyclic complexes of cobalt(II),” Asian J. Chem., vol. 36, no. 5, pp. 1056–1060, 2024. doi: 10.14233/ajchem.2024.31358.
C. Maurya et al., “Synthesis, characterization and antibacterial studies of new organotellurium carboxylates,” Afr. J. Bio Sci., vol. 6, pp. 263–276, 2024. doi: 10.33472/AFJBS.6.Si.2.2024.263-276.
S. M. Maaz, S. Bajpai, A. Singh, N. Srivastava, and G. Pandey, “Synthesis, characterisation and in-silico studies of novel heterocyclic organotellurium dithiocarbamates,” Res. J. Chem. Environ., vol. 25, p. 5, 2021.
C. Maurya, S. Bajpai, P. K. Kushwaha, U. Chand, and S. Singh, “Synthesis, spectroscopic characterization, and in vitro anti-microbial study of novel organotellurium (IV) diphenyldithiocarbamate derivatives,” J. Sulfur Chem., vol. 45, no. 4, pp. 477–489, 2024. doi: 10.1080/17415993.2024.2360441.
P. C. Srivastava, S. Bajpai, C. Ram, R. Kumar, and R. J. Butcher, “Synthesis, spectral and structural characterisation of ditel-luroxanes: μ-Oxo-bis[Nitrato-; 2,4,6-trinitrophenolato-dialkyl Tellurium (IV)],” J. Organomet. Chem., vol. 692, no. 12, pp. 2482–2490, 2007. doi: 10.1016/j.jorganchem.2007.02.026.
P. C. Srivastava et al., “C(sp3)H⋯O and C(sp2)H⋯O hydrogen bonds in acyclic- and cyclic-organotellurium carboxylates,” J. Organomet. Chem., vol. 649, no. 1, pp. 70–77, Apr. 2002. doi: 10.1016/S0022-328X(02)01124-5.
P. C. Srivastava, S. Bajpai, R. Lath, and R. J. Butcher, “Secondary bonds induced supramolecular assemblies in the crystals of 1,1,2,3,4,5-hexahydro-1,1-diiodotellurophene; 1,1,2,3,4,5,6-heptahydro-1,1-diiodotellurane and 1,3-dihydro-2λ4-benzotellurole-2,2-diyl diiodide,” J. Organomet. Chem., vol. 608, no. 1–2, pp. 96–105, Aug. 2000. doi: 10.1016/S0022-328X(00)00379-X.
P. C. Srivastava, S. Bajpai, and R. J. Butcher, “Synthesis, spectroscopic characterisation of 1,1,2,3,4,5-hexahydro-1,1-dicarboxylatotellurophenes and crystal structures of 1,1,2,3,4,5-hexahydro-1,1-di(benzoato)- and 1,1-di(4-nitrobenzoato)tellurophene,” J. Organomet. Chem., vol. 586, no. 2, pp. 119–124, 1999. doi: 10.1016/S0022-328X(99)00249-1.
P. C. Srivastava, A. Sinha, S. Bajpai, H. G. Schmidt, and M. Noltemeyer, “Dimethyl tellurium (IV) derivatives: synthesis, spectroscopic characterisation and structures of Me₂TeBr₂ and Me₂Te(OCOC₆H₅)₂,” J. Organomet. Chem., vol. 575, no. 2, pp. 261–268, 1999. doi: 10.1016/S0022-328X(98)01003-1.
P. C. Srivastava, A. Sinha, and S. Bajpai, “Synthesis and characterisation of tetra-, hexa-, octa-iodo and trihalo dimethyl tellurate (IV) derivatives,” Indian J. Chem. - Sect. A, vol. 38A, no. 7, pp. 727–729, 1999.
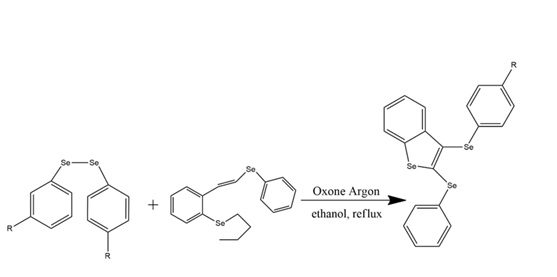
G. Perin et al., “Synthesis of 2,3-bis-organochalcogenyl-benzo[B]chalcogenophenes promoted by Oxone®,” New J. Chem., vol. 43, no. 16, pp. 6323–6331, 2019. doi: 10.1039/c9nj00526a.
D. Y. Direnko, Y. B. Drevko, and B. I. Drevko, “The synthesis of new organoselenium heterocyclic compounds: 2-aryl-4-phenyl-5,6,7,8-tetrahydro-4H-selenochromenes,” J. Chin. Chem. Soc., vol. 62, pp. 1068–1071, 2015. doi: 10.1002/JCCS.201500406.
A. D. Sonawane, Y. Kubota, and M. Koketsu, “Iron-promoted intramolecular cascade cyclization for the synthesis of sele-nophene-condensed, quinoline-based heteroacenes,” J. Org. Chem., vol. 84, pp. 8602–8614, 2019.
C. C. Tran, S. Kawaguchi, F. Sato, A. Nomoto, and A. Ogawa, “Photoinduced cyclizations of o-diisocyanoarenes with organic diselenides and thiols that afford chalcogenated quinoxalines,” J. Org. Chem., vol. 85, pp. 7258–7266, 2020.
G. Leonel, D. F. Back, and G. Zeni, “Synthesis of 3-substituted chalcogenophene-fused indoles from 2-alkynylindoles,” Ad-vanced Synthesis & Catalysis, vol. 362, pp. 585–593, 2020. doi: 10.1002/adsc.201901159.
Downloads
Published
How to Cite
CITATION COUNT
Issue
Section
License
Copyright (c) 2025 Shreya Dwivedi, Dr. Sangeeta Bajpai

This work is licensed under a Creative Commons Attribution 4.0 International License.



























