Studies of Some Aromatic Compounds for Corrosion Inhibition of Aluminum in Acidic Medium

DOI:
https://doi.org/10.54060/jase.v2i2.37Keywords:
Aluminium, inhibitors, inhibition efficiency, Tafel plot, weight lossAbstract
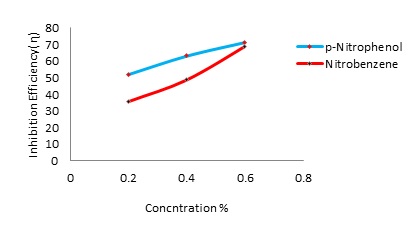
The aim of this work is to investigate corrosion inhibition characteristics of Alumi-num in Hydrochloric acid (0.1N) in presence of different concentration(0.2, 0.4% and 0.6%) of two inhibitors namely p-Nitrophenol and Nitrobenzene using Tafel polariza-tion studies and weight loss methods. Open circuit potential, inhibition efficiency, corrosion current and corrosion rate have been determined in the absence and pres-ence of inhibitors at different concentrations such as 0.2, 0.4% and 0.6%.It is ob-served that the inhibition increases with the increase in concentration of the inhibi-tors in acidic media. The experimental data showed that the maximum inhibition performance could reach about 72-78% at the concentration of 0.6%. The inhibition efficiency of the p-Nitrophenol is found to be higher than that of Nitrobenzene.
Downloads
References
S. V. Verstraeten, L. Aimo, P. I. Oteiza, “Aluminum and lead: molecular mechanisms of brain toxicity”, Arch. Toxicol., Vol. 82, no. 11, pp789–802, 2008.
J. R. Davis, “Corrosion of aluminium and aluminium alloys”, ASM International, Ohio, pp-313, 1999.
K. Xhanari, M. Finsgar, “Organic corrosion inhibitors for aluminum and its alloys in acid solutions: a review”, RSC Adv., vol. 6, pp. 62833–62857, 2016.
K. Xhanari and M. Finˇsgar, “Organic corrosion inhibitors for aluminum and its alloys in chloride and alkaline solu-tions: A review”,” Arabian J. Chem, vol. 12, no. 8, pp. 4646–4663, 2019
M. A. Quraishi, R. Sardar, “Corrosion inhibition of mild steel in hydrochloric acid by some aromatic hydrazides”, Materials Chemistry and Physics, vol.71, no. 3, pp. 309-313, 2001.
I. Lukovits, E. Kalman and F. Zucchi, “Corrosion Inhibitors—Correlation between Electronic Structure and Efficiency,” Corrosion, vol. 57, pp.3-81, 2001.
R. Johnson, J. T. Kakkassery, V. R. Palayoor, R. Kooliyat, and K. T. Vidhya, “Experimental and Theoretical Investiga-tions on the Corrosion Inhibition action of Thiadiazole Derivatives on Carbon Steel in 1M HCl medium”,” Oriental Journal of Chemistry, vol. 36, no. 6, pp. 1179–1188, 2020.
K. K. Alaneme, Y. S. Daramola, S. J. Olusegun, and A. S. Afolabi, “Corrosion Inhibition and Adsorption Characteristics of Rice Husk Extracts on Mild Steel Immersed in 1M H2SO4 and HCl Solutions,” Int. J. Electrochem. Sci, vol. 10, no. 4, pp. 3553–3567, 2015.
S. Karthikeyan, P. A. Jeeva, X. Hu, S. Harikumar, and S. Narayanan, “The Influence of Macro thiourea Derivative on the Cor-rosion of Mild Steel in Marine Environment”,” Oriental Journal Of Chemistry, vol. 28, no. 3, pp. 1443–1448, 2012.
X. Li, S. Deng, and H. Fu, “Synergistic inhibition effect of 6-benzylaminopurine and iodide ion on the corrosion of cold rolled steel in H3PO4 solution”,” Corrosion Science, vol. 53, no. 11, pp. 3704–3711, 2011.
R. Revie, Uhlig’s corrosion handbook, vol. 51. John Wiley & Sons, 2011.
M. G. Fontana, R. W. Staehel (eds), “Advance in Corrosion Science and Technologies”, New York: Plenum Press, vol. 6, 1976.
F. A. Champion, “Corrosion Testing Procedure,” Corrosion Testing Procedure, vol. 190, 1952.
P. S. Desai and R. T. Vashi, “Efficiency of Xylenol orange as corrosion inhibitor for aluminium in trichloroacetic ac-id”,” Indian Journal of chemical Technology, vol. 17, pp. 50–55, 2010.
A. K. Satapathy, G. Gunasekaran, S. C. Sahoo, A. Kumar, and P. V. Rodrigues, “Green corrosion inhibitors - An over-view,” Corrosion science, vol. 51, no. 12, pp. 2848–2856, 2009.
G. Singh, M. Sharma, and J. Chawla, “Cetyl trimethylammonium bromide as corrosion inhibitor for mild steel in acidic me-dium”, Indian J. Chem. Tech, vol. 16, pp. 339–343, 2009.
L. Li, X. Zhang, J. Lei, J. He, S. Zhang, and F. Pan, “Adsorption and corrosion inhibition of Osmanthus fragran leaves extract on carbon steel”,” Corrosion Science, vol. 63, pp. 82–90, 2012.
I. B. Obot and N. O. Obi-Egbedi, “Ginseng Root: A new Efficient and Effective Eco-Friendly Corrosion Inhibitor for Alu-minium Alloy of type AA 1060 in Hydrochloric Acid Solution,” Int. J. Electrochem. Sci, vol. 4, pp. 1277–1288, 2009.
D. Q. Zhang, L. X. Gao, and G. D. Zhou, “Inhibition of Copper Corrosion in Aerated Hydrochloric Acid Solution by Amino-acid Compoundˮ,” J. Appl. Electrochem, vol. 46, pp. 1081–1085, 2005.
T. Vashi, R., H. M. Bhajiwala, and S. A. Desai, “Ethanolamines as corrosion inhibitors for Zinc in (HNO3+ H2SO4) binary acid mixture”,” E-Journal of Chemistry, vol. 7, no. 2, pp. 665–668, 2010.
X. Zheng, S. Zhang, W. Li, M. Gong, and L. Yin, “Experimental and theoretical studies of two imidazolium-based ionic liquids as inhibitors for mild steel in sulfuric acid solution”,” Corrosion science, vol. 95, pp. 168–179, 2015.
H. Ashassi-Sorkhabi, B. Shaabani, and D. Seifzadeh, “Corrosion inhibition of mild steel by some Schiff base com-pounds in hydrochloric acid”,” Applied Surface Science, vol. 239, no. 2, pp. 154–164, 2005.
Downloads
Published
How to Cite
CITATION COUNT
Issue
Section
License
Copyright (c) 2022 shanker Lal Meena, Lal chand Yadav

This work is licensed under a Creative Commons Attribution 4.0 International License.



























